‡T
. Factors Influencing the Formation of a Mousse‡U. Investigation of the Effect of Waves on the Formation of a Mousse
‡V
. Investigation of the Effect of Emulsifiers on the Formation of a MousseЃ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Ѓ@
Crude oil after being spilled from a wrecked oil tanker takes in sea water under the influence of wind and waves, and tends to form a highly viscous oily matter, usually called a "mousse", which contains a high sea water content. A technology for efficiently recovering this mousse oil needs to be developed. To do this, it is necessary to clarify the mechanism of how the characteristics of spilled oil change with time, the so-called "formation of a mousse".
In a project for developing techniques for combating large-scale oil disasters, we carried out an experimental investigation of the changes of spilled oil with time. This article is a summary of those studies on clarifying the mechanism of the formation of a mousse.
Incidentally, these studies were carried out on the two premises that the formation of mousse is a phenomenon which occurs in some short time , and because those oils of high fluidity point or light gravity don't form a mousse , we didn't deal with them.
Return to Index . Factors Influencing the Formation of a Mousse (from Crude Oil) ЃD Temperature1Obviously, seasonal factors (temperature, humidity, luminosity, and the intensity of ultraviolet light) affect the "formation of a mousse." However, these factors except for temperature don't need to be considered for a mousse that is formed in several days.
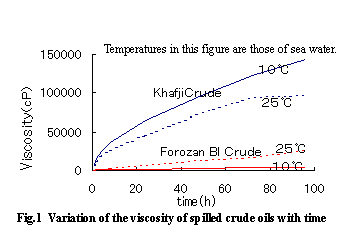
Temperature has a major effect on the viscosity of crude oil spilled at sea. The viscosity in turn affects the ease of mixing of sea water which causes the formation of a mousse. The tendency of spilled crude oil to form a mousse caused by temperature depends not only on the composition but also the viscosity of the oil at a given temperature. Increasing temperature may accelerate the formation of a mousse (high water content and viscosity) for certain oils or may retard it for other oils.
Figure 1 shows the viscosity with time under constant wave conditions for two types of crude oil, obtained in the experiments using a water tank.
Ѓ@
Crude oil having a fluidity point far above the seasonal variation range of temperature only tends to coagulate to form an oily mass and not a mousse.
Return to Index ЃD Sea WavesSimple contact of crude oil with sea water without motion may cause little emulsification. The mixing energy of sea waves plays a substantial role in the formation of a mousse.2 Waves are the motion that results from the repeated circular motions of sea water in a vertical direction caused by the wind. Figure 2 shows the formation of waves due to the circular motions of sea water.
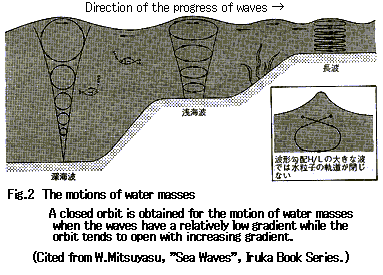
When crude oil is spilled at sea, it is mixed with sea water by the circular motion of the sea water. The continuous process of this mixing causes the formation of a mousse. Increasing energy of waves increases the speed of formation of a mousse. However, waves having excessively high energy (such as in a storm) will cause the dispersion of oil in sea water and a mousse will not be formed.
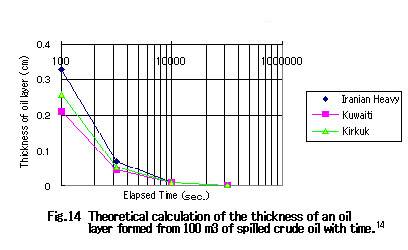
Return to Index ЃD Thickness of Oil LayersIf the fluidity point of a spilled crude oil is higher than the temperature of the sea water in the area of the accident, then the oil is cooled by the sea water and coagulates. Thus, a thin oil layer will not be formed and instead the coagulated matter will float on the sea. If the fluidity point of a crude oil is lower than the temperature of the sea water, the oil will spread over the sea as a thin film immediately after the spillage. In reference 3, the thickness of the oil layer with time was calculated for a 100 m3 spill of Iranian heavy crude oil and Kuwaiti crude oil, respectively, using the expression for P.C. Blokker's diffusion model. The results are given in Table 1. It shows that crude oils having a relatively low fluidity point such as Iranian heavy crude oil tend to form an oil layer of thickness less than 1 mm in 10 minutes or more after the spillage.
|
Elapsed Time Oil Name |
100sec |
1,000sec |
10,000sec |
100,000sec |
|
Iranian Heavy Crude |
0.327 |
0.070 |
0.011 |
0.003 |
|
Kuwaiti Crude Ѓ@ |
0.210 |
0.045 |
0.010 |
0.002 |
Table 1 Thickness (cm) of oil layer as a function of time after spillage of 100 m3 of crude oil
Ѓ@
For crude oils having a low fluidity point, the thinner the oil layer, the more easily mousse tends to be formed, due to the ease of mixing. As shown in Table 1, a thin oil layer is usually expected to be formed at sea in a short time. Correspondingly, a mousse is likely to be formed easily.
The spreading of spilled oil over the sea surface calms the waves and so the mousse will be formed more slowly from the surface to the center of the oil layer with depth. This suggests that the prevention of spreading and exposure to waves by using oil fences as soon as possible may effectively inhibit the formation of a mousse.
Return to Index ЃD Emulsifiers in Crude OilA mousse is formed through the formation of a W/O-type emulsion by the intake of sea water by crude oil. The formation of this W/O-type emulsion requires an emulsifying agent, in other words, an emulsion stabilizer that prevents sea water from escaping. Asphaltene, resin, and wax in crude oils are considered to act as emulsifiers. Asphaltene and resin are actually involved in the formation of a mousse oil.
Asphaltene is a hard, brittle brown or black powder with a mean molecular weight of approximately 2,000. Asphaltene in a crude oil exists as a micelle, a sort of colloid, of asphaltene in maltene (resin + oil). This emulsifying ability of asphaltene may be ascribed to a mechanically protecting effect, that is, the formation (Fig. 3) 4 of a mechanically strong membrane by the adsorption of asphaltene particles on water drops surface rather than to a surface-active effect.
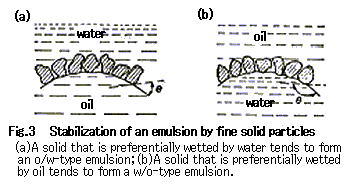
Resin is an extremely viscous, black liquid with a mean molecular weight of approximately 1,000 and is composed of long-chain alkyl groups and polycondensed aromatic rings. It is diluted with lower hydrocarbons in crude oil and has a substantial influence on the viscosity of crude oil. The emulsifying effect of resin may be principally attributed both to a surface-active effect and to the viscosity of the resin itself.
Return to Index . Investigation of the Effect of Waves on the Formation of a Mousse ЃD Mixing of Crude Oil and Sea WaterThe spillage of crude oil at sea containing a relatively large amount of heavy fractions may lead to the formation of a mousse oil with time. Mixing of crude oil and sea water occurs under the action of waves in the spillage area, followed by the intake of sea water by the oil to form a W/O emulsion that has a high viscosity. Although this mixing effect of waves plays an essential role in the formation of mousse, the mechanism of the intake of sea water by crude oil remains unclear because of its instantaneous process and the difficulty of observation by the naked eye. We therefore investigated the process of the inclusion of sea water in crude oil as well as that of the transfer of crude oil from the surface of the sea to the bulk of sea water by using a high-speed video camera. Analysis was performed by close examination of each image.
Qatar Marine crude oil was poured onto the surface of sea water in a circulating tank in which broken waves were generated intermittently. The oil sank just below the surface, then floated and spread out over the surface. Part of the oil was divided into drops and suspended in the water. The size of the oil drops ranged from about 1 cm down to a few millimeters. This behavior of the oil is due to a circular motion in the vertical direction by waves. If the buoyancy of the oil exceeds the kinetic energy of the water mass, it can float, but otherwise it breaks up into drops and is suspended in water.
Photograph 1 shows a side view of this stage. The following relationship giving the boundary conditions for the formation of oil drops from an oil layer has been reported.5
[Intensity of Turbulence]
Ѓ„[Buoyancy]Ѓ{[Surface Tension]Ѓ@

Ѓ@
Photograph 1After a few minutes the oil layer had spread out, broken waves disappeared and oil drops in the water began to float to the surface and then larger oil drops gathered just below the oil layer floating on the surface of the sea water. Some of the oil drops that had gathered were taken in by the oil layer and the rest drifted with the motion of the waves. Photograph 2 shows this stage.
Ѓ@

Ѓ@
Photograph 2Ѓ@
Photographs 3-6 show the sequential appearances of the intake of the oil by the sea water under the action of waves.
Ѓ@
Photograph 3
Photograph 4
Ѓ@
Photograph 5
Ѓ@
Photograph 6Ѓ@As seen in these photographs, the oil near the surface of the sea water tends to gather around the wave crests and travels in the direction of the progress of the waves. This causes the unevenness in the thickness of the layer. In the breaking process of the wave crests, it is also shown the oil moves in the vertical direction under vigorous stirring by waves and thus is dispersed into the sea water to form oil drops.
The captured oil drops slowly float to the sea surface due to buoyancy and coalesce again to form emulsified crude oil at the surface of the sea water. In the case of oil that forms a mousse easily, repeating this process leads to the formation of a mousse oil with the intake of the sea water. In contrast, in the case of crude oil that does not easily form a mousse, it tends to disperse in water.
Return to Index ЃD Intake of Sea Water by Crude OilWe generated calm waves in a water tank on a laboratory bench, then poured the crude oil ontohe surface of the sea water to form a thin layer of oil and we observed the intake of sea water by the oil. Photograph 7 shows the oil layer thus formed from Iranian heavy crude oil.
Ѓ@
Photograph 7Then, we generated waves of increasing intensity and observed the motion of the oil layer. The layer spread out to form an uneven thinner layer. Towards the beginning of the emergence of turbulent flow on the surface of the sea water, the oil layer became thicker at the top of waves and thinner at the bottom. The size of particularly thick scattered oil layers was several millimeters. During the extending and shrinking by the wave action, this layer was transformed into a crescent that was open in the direction opposite to the progress of waves and in the next moment it surrounded water drops of 1 mm or so in diameter. This phenomenon occurred here and there, and the oil drops gradually coalesced to form an emulsion. Photograph 8 shows the sequence of this process. Throughout this process, intake of the oil drops into the sea water was not observed because of the calm waves.
The results described above suggest the following mechanism for the formation of a mousse under the influence of waves.
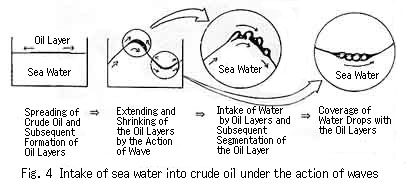
a). Spilled crude oil first spreads out over the sea surface to form oil layers, which repeatedly extend and shrink under the action of waves.
b). A thick oil layer develops at the interface between briskly moving sea water and an oil layer which extends and shrinks repeatedly. In this way the mixing of water and oil occurs near the wave crests. The water that enters the oil layer tends to form a spherical shape due to surface tension.
c). Adsorbates in the crude oil are immediately adsorbed on the surface of the water drops. The adsorption layer then stabilizes these water drops by preventing water from escaping.
The repetition of processes b) and c) leads to the formation of an emulsion by the intake of sea water by crude oil and finally to the formation of a mousse oil.
Ѓ@
Ѓ@
Photograph 8Figure 4 shows the process of the intake of sea water by crude oil on the basis of the present results. Considering that emulsification can occur without the formation of oil drops by broken waves, we can conclude that the extending and shrinking of an oil layer by wave action may play the most important role in the intake of sea water by crude oil. We can also conclude that, in the case of crude oils which tend to form a mousse easily, the increased surface area through the formation of oil drops by the action of broken waves may facilitate the intake of sea water. In the case of light crude oils, which do not easily form a mousse oil, the dispersion of oil into sea water may be promoted.
Ѓ@
Ѓ@
Return to Index . Investigation of the Effect of Emulsifiers on the Formation of a Mousse ЃD Effect of Asphaltene, Resin, and Wax on the Formation of a MousseThe magnitude of the effect of asphaltene, resin, and wax on the formation of a mousse decreases in this order. Table 2 shows the characteristics of test oils that were prepared by mixing an equal amount of asphaltene, resin, or wax isolated from Kuwaiti crude oil with lubricant-base oil, and also those of the mousse oil formed from those test oils.
Table 2
|
|
Asphaltene+ Lubricant-base oil |
Resin+ Lubricant-base oil |
Wax+ Lubricant-base oil |
Lubricant-base oil |
|
|
Characteristics of Test oil Ѓ@Ѓ@ Ѓ@ Ѓ@ Ѓ@ |
Ѓ@ Density (g/cm3)@15ЃЋ |
Ѓ@ 0.9469 |
Ѓ@ 0.9263 |
0.9024 |
0.9077 |
|
Viscosity (cP) Ѓ—15ЃЋ |
1190 |
261 |
89.6 |
60.9 |
|
|
Water content ( v%) |
0.0 |
0.0 |
0.0 |
0.0 |
|
|
Surface tension Ѓidyne/cm) @15ЃЋ |
30.5 |
30.7 |
29.6 |
30.8 |
|
|
Interfacial tension Ѓidyne/cm) @15ЃЋ |
26.4 |
27.8 |
27.1 |
28.1 |
|
|
Ѓ@ |
|||||
|
Characteristics of mousses from each test oil |
Density (g/cm3)@15 ЃЋ |
0.9823 |
0.9624 |
0.9640 |
0.9080 |
|
Viscosity (cP) Ѓ—15ЃЋ |
22100 |
3120 |
425 |
61.0 |
|
|
Water content ( v%) |
78.0 |
74.0 |
76.2 |
0.5 |
|
Ѓ@
The effect of asphaltene was striking. The increase in viscosity with asphaltene was about ten times higher than that with resin and about a hundred times higher than that with wax. All of the test oils were able to take in water to saturation. However, after a while, the test oil containing wax easily released the water that had been absorbed because of the instability of the emulsion that had formed.
Return to Index ЃD Effect of Asphaltene, Resin, and Wax on the Interfacial TensionWe examined the mousse oil prepared from the test oils described above, using an electron microscope. The mousse oil containing asphaltene exists as a stable W/O emulsion of which the oil layer surrounds water droplets of about 1 micron in diameter. A similar result was obtained in the mousse containing resin. Its stability was inferior to that of the mousse oil containing asphaltene and gradual coalescence of water droplets was observed. In the case of the mousse oil containing wax, water droplets of dozens of microns, much larger than those in the mousse containing asphaltene or resin, were observed. In addition, the repeated coalescence of particles followed by rapid separation of oil and sea water were observed. In summary, the different size and stability of water droplets in the different emulsions are attributable to the difference in the function of asphaltene, resin, and wax as emulsifiers.
The emulsifying effect is divided into two types:
a) Lowering of the interfacial tension between oil and water due to the surface-active effect, and
b) Formation of a mechanically protecting layer through the adsorption of macromolecules at the interface.6,7,8
To examine whether the former effect occurs or not, we measured the interfacial tension between the test oil and sea water in the presence of these emulsifiers. In all cases, no significant lowering of the interfacial tension was observed. A larger decline of the interfacial tension was found for wax which has the weakest emulsification power. The surface tension for these test oils and their mousse oils are shown in Table 2. From the data of this table, we may conclude that a mousse forms through the interfacial absorption of macromolecules such as asphaltene, resin and wax (the second type), because there is no relation between the surface tension of the emulsifiers tested and the ease of formation of a mousse.
Return to Index ЃD Relation Between the Amount of Asphaltene and the Formation of a MousseTo understand the relation between the amount of asphaltene and the formation of a mousse from crude oil, we isolated asphaltene from a variety of crude oils and then tested it for the formation of a mousse by dispersing various amounts of the asphaltene in the lubricant-base oil. As seen in Figure 5, initially the viscosity of the mousse oil gradually increased with increasing amount of the asphaltene. Then, a rapid increase was observed at concentrations higher than 3-5 wt% of asphaltene.
.gif)
Fig.5
Ѓ@
This phenomenon may be closely related to the formation of a mechanically protecting layer through the interfacial adsorption of the macromolecules.
In general, asphaltene is considered to exist partly in the dissolved state and partly in a micelle in which asphaltene exists as a dispersion in maltene (resin and oil). 9 Therefore, the change in the composition owing to the removal of maltene from, or the addition of a large amount of less-saturated hydrocarbons such as pentane, to crude oil may lead to the coagulation and subsequent deposition of micelles. The present study was carried out under the conditions where an increasing amount of asphaltene led to a lack of the dispersing medium. If the amount of added asphaltene exceeds the dispersing capacity of oil, it will not be able to exist any more in micelles (an associated state) and will deposit as fine solid particles. In other words, asphaltene can exist in a dissolved or dispersed state below the concentration of 3-5 wt%, but beyond this threshold it separates out into fine particles. These particles are adsorbed on the surface of water drops to form a mechanically protecting layer 10 and thus a stable emulsion is produced.
Return to Index . Investigation of the Effect of Dispersing Media on EmulsifiersWe isolated asphaltene, resin, and wax from Khafji crude oil and prepared test mixtures by dispersing a given amount of each of these emulsifiers in two types of solvents (n-decane and n-butylbenzene) 11 which have different dissolving abilities. For these test mixtures, the viscosity and water contents were measured before and after the formation of a mousse.
Figure 6 shows the viscosity and water contents before and after the formation of a mousse for the test oil prepared by adding asphaltene to n-decane and n-butylbenzene. The test oil containing n-butylbenzene only gave an unstable soft emulsion whereas the oil containing n-decane gave a stable mousse oil. This fact clearly indicates that the formation of a mousse depends on the nature of the solvent.
.gif)
Ѓ@
Photograph 9 shows dispersions prepared by mixing asphaltene with n-decane and n-butylbenzene respectively as observed with a microscope.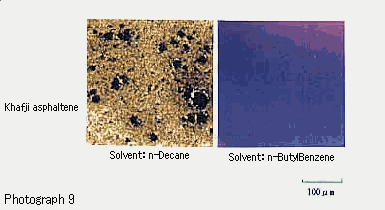
As seen in Photograph 9, very different patterns of dispersion are obtained with the solvents of different dissolving abilities. Thus, asphaltene is dispersed in the form of fine solid particles in n-decane, but is soluble in n-butylbenzene.
Figure 7 shows the viscosity and water contents before and after the formation of a mousse for the test oil prepared by adding resin to n-decane and n-butylbenzene. In both solvents, a stable mousse oil was obtained. The difference in viscosity between both mousse oils was small compared with that observed for asphaltene. This means that the resin has a smaller effect as the dispersing medium.
.gif)
Figure 8 shows the viscosity and water contents before and after the formation of a mousse for the prepared test oil by adding wax to n-decane and n-butylbenzene. In both solvents, only an unstable emulsion was obtained. No difference was found in the formation of mousse between both solvents. However, a larger intake of water was observed in n-decane than in n-butylbenzene.
.gif)
As seen in the results above, the nature of solvents influences the formation of a mousse more significantly for asphaltene. The saturated hydrocarbon having a lower solvent ability promotes the formation of a mousse more effectively. Asphaltene works better as fine solid particles in a solvent. Resin exhibits a behavior similar to that of asphaltene and promotes the formation of a mousse in n-decane more effectively than in n-butylbenzene. Although only an unstable emulsion is formed with wax, the intake of water was larger in n-decane than in n-butylbenzene. This fact can also be attributed to the dissolving ability.
Asphaltene as fine solid particles is the best promoter of the formation of a mousse among emulsifiers that stabilize a W/O-type emulsion. This fact is attributed to its great tendency to absorb at the interface; thus, the fine particles of asphaltene adsorb on the water drops to form a mechanically protecting layer.12 Asphaltene in a dissolved state (micelle and colloid) is inferior in the formation of a mousse to that as solid fine particles. However, it can stabilize a mousse in higher concentrations. This can be explained in terms of the increasing effect of micellar or colloidal asphaltene on the viscosity of the solution as well as the stabilization of a W/O-type emulsion by the adsorption of the grown micelles/colloids on water drops.
Although resin cannot exist as solid fine particles, its high viscosity and its surface activity may somehow contribute to preserving the water drops.
Wax does not contribute in the liquid state to the stabilization of the W/O-type emulsion, because it has not only a low surface activity, but also a very low viscosity in the liquid state. However, wax partly dissolves and partly disperses as fine particles in n-decane and thus contributes little to the stabilization of the emulsion due to the interfacial adsorption like asphaltene in n-decane.
Return to Index . Investigation of the Cooperative Effect of Combined Emulsifiers.To study the cooperative effect of combined emulsifiers on the formation of a mousse, we isolated asphaltene, resin, and wax from Khafji crude oil and prepared the test oil by dispersing them individually or in combinations in a constant total amount in n-decane. The results of the test are shown in Fig. 9.
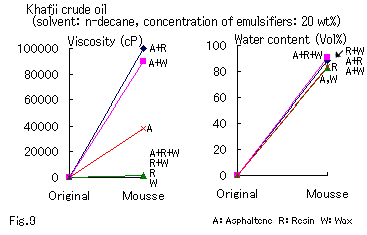
A synergistic effect on the viscosity was observed in asphaltene + resin and asphaltene + wax. Thus, these combinations of emulsifiers promoted the formation of a mousse. Among the combinations examined, the synergistic effect of the combination of asphaltene with resin in n-decane may be due to the presence of asphaltene in solid particles and to the high viscosity of resin, which may increase the binding force between the asphaltene particles.
It is known that fine solid particles tend to come together at the interface between oil and water and can contribute to the stabilization of an emulsion.13 The formation of mousse oil in the presence of asphaltene may be accomplished through the adsorption of the fine particles of asphaltene on the water drops. The mutual association of the adsorbed asphaltene is not so strong as its adsorption on the water particles. However, it allows resin to enter the space between the asphaltene particles to strengthen their mutual association. Thus, the mousse oil can be stabilized.
The nature of the interaction between asphaltene and wax is not known, but it may be related to the coexistence of the particles of both asphaltene and wax.
Return to Index . Investigation of the Molecular Structures of EmulsifiersWe isolated asphaltene, resin, and wax from three types of crude oil (Duri, Khafji, and UmmShaif) and carried out structural analyses. The following characteristics were obtained:
Asphaltene is a mixture of high-molecular hydrocarbons with rich aromaticity. The index of aromaticity, fa, which is a parameter of average molecular structure, was 0.36 for asphaltene from Duri crude oil, 0.54 for that from Khafji crude oil, and 0.53 for that from UmmShaif crude oil. The magnitude of aromaticity of Khafji, UmmShaif, and Duri crude oils decreases in this order.
Figure 10 shows the average molecular structures presumed based on the structural analyses. As seen in these models, the asphaltene from Duri crude oil is characterized by alternate linkage of polycondensed aromatic rings and long-chain hydrocarbons. The asphaltene from Khafji crude oil has a stacked structure of polycondensed aromatic rings and short-chain hydrocarbons alternately linked with each other. The asphaltene from UmmShaif crude oil has a lower molecular weight than the other two. Its structure is similar to that of asphaltene from Duri crude oil because its asphaltene also contains polycondensed aromatic hydrocarbons and short-chain hydrocarbons that are alternately connected with each other
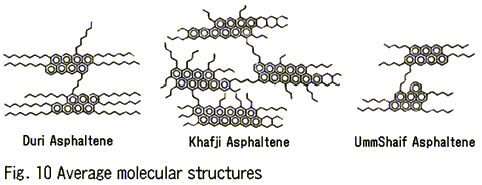
Fig. 11 shows the average molecular structures of the resins. These three types of resins have low aromaticity compared with that of the asphaltenes. Each of the resins contains condensed aromatic rings, long-chain hydrocarbons, and naphthenic rings; the last being connected with the condensed rings.
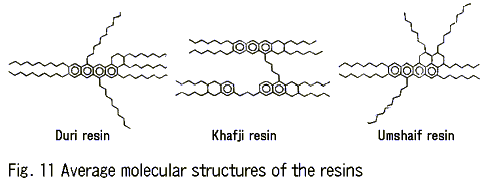
The waxes are composed exclusively of saturated hydrocarbons with long chains. Polycondensed aromatic rings are not contained in these waxes in contrast with the asphaltenes and the resins.
Considering the relations between the molecular structures of the emulsifiers and their effect on the formation of a mousse, we can conclude that emulsifiers having the stacked high-molecular structure of polycondensed aromatic rings and short open-chain hydrocarbons contribute to the formation of a mousse to a greater degree.
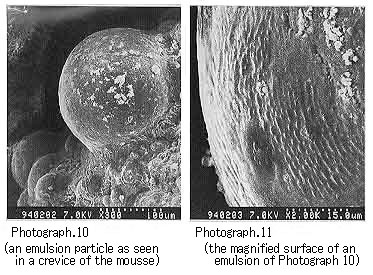
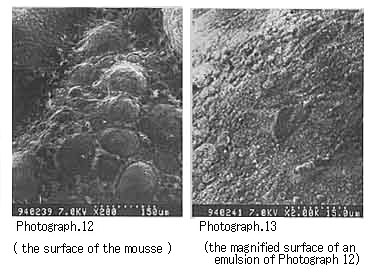
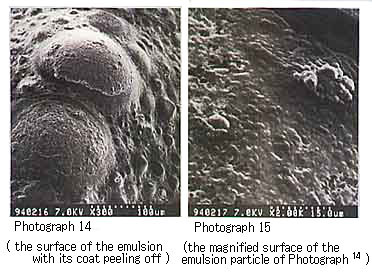
Return to Index . Electron Microscopic Observation of Emulsion Particles in a MousseWe dispersed in n-decane each of the emulsifiers isolated from a variety of crude oils and then prepared mousses. We then observed the emulsion of the mousse oils with an electron microscope. For the mousse oils prepared by dispersing in n-decane each of the asphaltenes isolated from Khafji, UmmShaif, and Duri crude oils respectively, we were able to observe the asphaltene particles (several microns in diameter) that covered the water particles (Photographs 10 to 15).
Photograph 10 and Photograph 11 shows mousse oil with Khafji asphaltene.
Photograph 12 and Photograph 13 shows mousse oil with UmmShaif asphaltene.
Photograph 14 and Photograph 15 shows mousse oil with Duri asphaltene.
In the mousse oils using each of the resins from Khafji and UmmShaif crude oils respectively, we were able to observe the water particles multiple-surrounded with the resin (Photographs 16 to 18).
Photograph 16 and Photograph 17 shows mousse oil with Khafji resin.
Ѓ@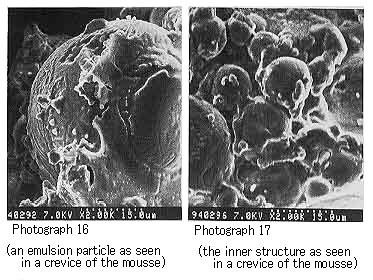
Photograph 18 shows mousse oil with UmmShaif resin.
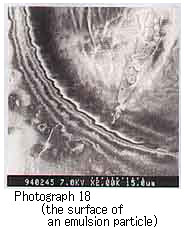
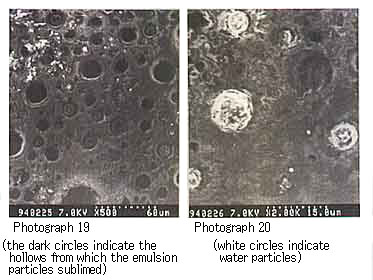
For the mousse oil using the resin from Duri crude oil, water particles or hollows were observed; the water particles were those left after the resin sublimed during electron microscope observation, and the hollows were left after the water particles had sublimed (Photographs 19 and 20).
Photograph 19 and Photograph 20 shows mousse oil with Duri resin.
We were able to observe a mousse oil similar to that obtained with Duri resin (Photographs 21 and 22).
Photograph 21 and Photograph 22 shows mousse oil with Duri wax.
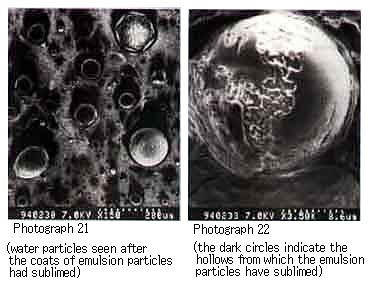
These photographs show the formation of characteristic mousse oils as a function of the constituents of each crude oil. From these microscopic photographs as well, we can confirm that the stable mousse oils are formed by the water droplets surrounded with asphaltene or resin particles.
Return to Index . Investigation of the Effect of the Residues Obtained after Removal of the Emulsifiers from Crude Oils on the Formation of a MousseSince we were able to identify asphaltene, resin, and wax as substances causing the formation of a mousse, we next investigated the effect of constituents other than these three substances. Table 3 compares the effect on the formation of a mousse between Arabian heavy crude oil (this oil is well known to have a great tendency to form a mousse) and its residual oil obtained after removal of the emulsifying substances.
Table 3
| Test oil |
Arabian heavy crude oil |
Residual oil obtained after removal of emulsifiers |
||||
|
Formation of a mousse |
Before |
After |
Before |
After |
||
|
Water content (Vol%) |
0.0 |
82.1 |
0.0 |
46.1 |
||
|
Viscosity at 15 ЯC (cP) |
116 |
33700 |
6.83 |
37.9 |
||
|
Density at 15 ЯC (g/cm3) |
0.9069 |
1.0081 |
0.8653 |
0.9523 |
||
Ѓ@
As seen in Table 3, the residual oil has no ability to form a mousse any more. In conclusion, the formation of a mousse is undoubtedly attributed to the heavy constituents such as asphaltene, resin, and wax. Constituents other than these substances are not responsible for the formation of a mousse. The heavy constituents themselves, however, are immiscible with sea water because of their extremely high viscosity and hence have no ability to form a mousse. Thus, non-heavy constituents also have no ability to form a mousse, but function as a dispersing medium to help the heavy constituents to form a mousse. These findings are summarized in Fig. 12.
The schematic mechanism of the formation of a mousse is shown in Fig. 13.
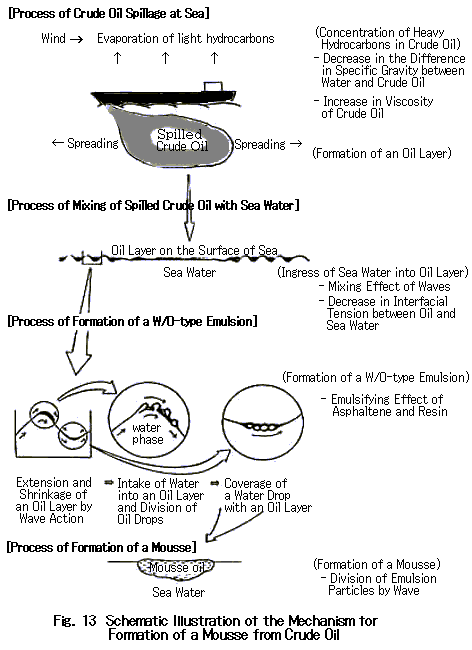
The process is composed of four stages. This section discusses each stage in detail.
ЃD Process of Spillage of Crude Oil at SeaCrude oils which spilled from tankers spread over the sea surface immediately and a thin layer is formed if the crude oil has a low fluidity point. More volatile constituents evaporate quickly and thus the less volatile substances concentrate. As suggested from the expression for Blokker's diffusion model, an oil layer as thin as 1 mm can form within ten minutes even if as much as 100 m3 of crude oil is spilled at once (Fig. 14).
On an actual sea, spilled oil rapidly spread under the accelerating influence of wind and waves, and also the tide. Evaporation of volatiles greatly depends not only on the temperature of the sea water and atmosphere, but also on the wind and waves in the area. It is known that in the seas surrounding Japan all year around, C7-C8 hydrocarbons are completely evaporated off within two hours after spillage from any kind of crude oil having any fluidity. This phenomenon occurs because the ease of evaporation of each hydrocarbon is determined by its saturated vapor pressure.
Figure 15 shows the dissipation of hydrocarbons for light crude oil and heavy crude oil as a function of time.
Ѓ@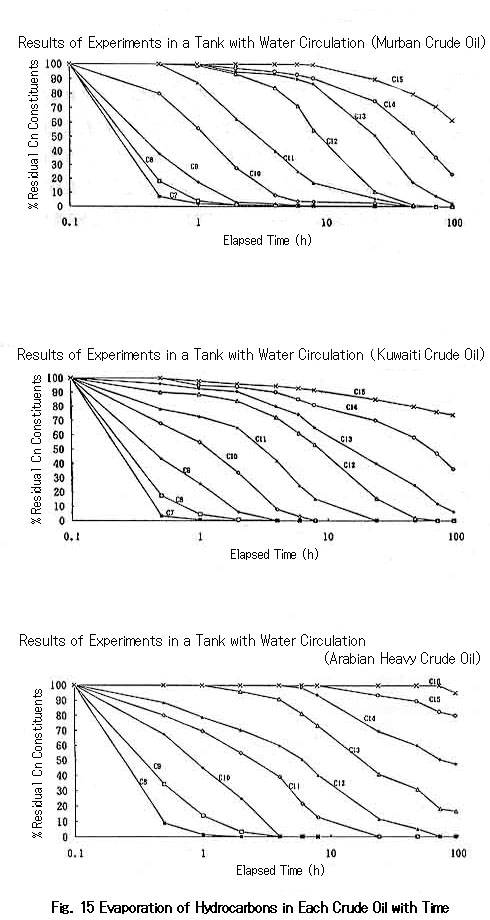
As seen in this figure, the dissipation of each hydrocarbon follows similar profiles irrespective of the volatility of both crude oils. Thus, the proportions of heavier constituents as well as the density, viscosity, and surface tension of spilled oil gradually increase with time; this causes a decrease both in the interfacial tension and in the difference in density between sea water and spilled oil.
In contrast with an oil having a low fluidity point, crude oils having a high fluidity point forms the oil balls soon after its spillage, and drift on the sea surface. A crude oil having a high fluidity point contains a high heavy wax content and is usually heated to maintain fluidity during transportation; therefore such a crude oil having a high fluidity point may solidify by exposure to low temperatures. This is why a crude oil having a high fluidity point does not form a mousse and forms the oil balls when spilled at sea. In the later discussion we will not deal with such a crude oil, because it has no tendency to form a mousse.
Return to Index ЃD Process of Mixing of Spilled Oil with Sea WaterConcurrently with process 1, spilled crude oil containing volatile constituents undergoes mixing with sea water under the influence of wind and waves through the ingress of sea water and its release from the thin oil layer, or the ingress of oil drops into the water and their floating. This process is promoted, under the action of waves, through the repeated expanding and shrinking of the layer on the sea surface and through the forced sinking and floating by the action of high rolling waves. (The mixture of oil and sea water is not stable at this stage and the two tend to separate from each other as soon as the sea calms down). As time passes, the spilled oil becomes heavier and heavier as the volatile constituents evaporate. Correspondingly, the release of captured water from an oil layer, and the sinking and floating of oil drops diminishes. A W/O-type emulsion begins to be formed although its viscosity is still low. Some emulsions may contain more than 70% of water.
ЃD Process of Formation of a W/O-type EmulsionIn Process 2, the sea water particles captured in an oil layer are so large that they can be seen by the naked eye and the emulsion is still unstable. In a W/O-type emulsion, a crude oil forms a continuous phase and sea water is dispersed in this phase. The very high viscosity and low surface tension of a crude oil compared with those of sea water lead to the formation of this W/O-type emulsion. However, these requirements themselves cannot provide a stable W/O-type emulsion. An emulsifying substance that surrounds water particles and prevents the latter from coalescing is required for a stable emulsion to be formed. As discussed above, asphaltene, resin, and wax as non-volatile constituents in crude oils serve as emulsifying agents. Asphaltene tends to form micelles in crude oils. When water is added to the crude oil, asphaltene is adsorbed by electrostatic attraction on the water particles to form a mechanically protecting layer, which prevents the water particles from coalescing. Resin prevents the coalescence of water particles as well and also maintains the space between each of the adsorbed asphaltene particles through weak surface activity and its own viscosity.
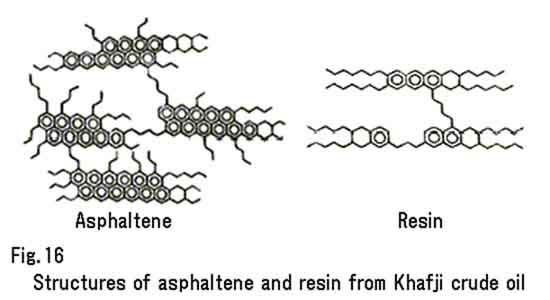
These emulsifiers start to work as soon as crude oil is spilled and mixed with water by waves. At an early stage, however, emulsification does not occur owing to the inhibitory action of the volatile constituents. As these substances progressively decrease, the adsorption effect of the emulsifiers becomes apparent, and correspondingly, a stable W/O emulsion begins to develop. The function of asphaltene and resin to form a stable W/O emulsion depends on their structure, which causes the difference in the interfacial activity. Asphaltene is a combination of macromolecular hydrocarbons having polycondensed aromatic rings and open-chain hydrocarbons that are linked with each other. Resin has a similar macromolecular structure with less aromatic rings and more long-chain hydrocarbons. These hydrocarbons having more aromatic rings show greater interfacial activity. Figure 16 shows the structures of asphaltene and resin from Khafji crude oil.
Fig. 16 Structures of asphaltene and resin from Khafji crude oil.
The stability of a W/O-type emulsion depends also on the contents of asphaltene and resin in the original crude oil. Crude oils containing a high content of these emulsifiers tend to give a stable emulsion, while those containing a low content have little tendency to form a stable emulsion. This is why crude oils containing high contents of volatile constituents generally do not provide a stable emulsion.
Return to Index ЃD Process of Formation of a MousseThe water content of an emulsion sharply increases at the initial stage of the formation of a stable W/O-type emulsion and then reaches a plateau. In contrast, the viscosity of crude oils continues to increase with time by three to four orders of magnitude compared with the initial viscosity. Figure 17 shows the water content and viscosity of Arabian heavy crude oil with time as obtained in experiments using a tank with water circulation. As seen in the figure, the water content sharply increased and reached a content as high as 70% in a short period of time and then leveled off. The viscosity slowly increased and reached a plateau of 20,000 cP. All crude oils that can form a stable W/O emulsion behaved similarly.
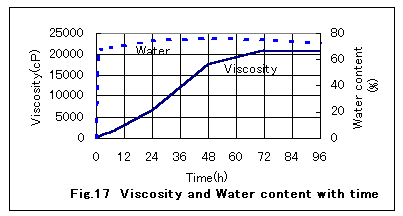
The gradual increase in viscosity is attributed to an increase in abrasion resistance produced between each of the oil layers surrounding fine water particles that are being formed by repeated division by wave action, of water drops enclosed in oil layers. De-emulsification of a mousse produces sea water and crude oil with concentrations of heavier hydrocarbons owing to the evaporation of volatile constituents. The crude oil thus recovered has quite a low viscosity compared with the mousse oil before de-emulsification.
Figure 18 shows electron microscopic photographs (at the same magnification) of the surface of an emulsion (at earlier and later stages) from Khafji crude oil.
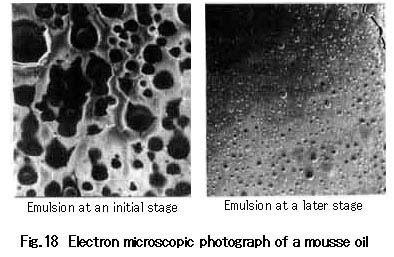
The photographs support the mechanism discussed above for the increase in viscosity of a W/O emulsion.
The emulsion at this stage is stable against phase separation owing to its high viscosity. It presents a dark brown color because of its high water content and looks like a chocolate mousse. It drifts as massive oil on the sea surface.
Return to Index Footnotes1. The temperatures in the present work are those of both the thermosphere and sea water whose temperatures change with the seasons.
2. Petroleum Association of Japan, "Survey of References on the Change of Spilled Oil with Time" in 1990.
3. Journal of the Institute of Petroleum, Vol.54, No.539.
4. A. Kitahara and K. Aoki, "Chemistry of Colloids and Interfaces", Third Edition.
5. Japan Ocean Industries Association, "Report of the Assessment of the Impact of Oceanic Petroleum Development on the Environment" (1984).
6. T. Kondo and S. Suzuki, "Science of Colloids and Interface" Second Ed., Sankyo Publ. Co.
7. Translated by A. Kitahara and K. Aoki, "Chemistry of Colloids and Interface" Third Ed., Hirokawa Shoten.
8. H. Suzuki, "Interface and Surface Active Substances", Sangyo Tosho.
9. K. Kanezaki and T. Okada, "Asphalt", Nikkan Kogyo.
10. Mark Bobra, "Physicochemical Study on the W/O-type Emulsification" (Report).
11. We chose n-decane as a saturated paraffinic hydrocarbon and n-butylbenzene as an aromatic hydrocarbon, because both compounds are liquid at room temperature and have the same number of carbon atoms, but different dissolving abilities.
12. A. Kitahara and K. Aoki, "Chemistry of Colloids and Interface" Third Ed., Hirokawa Shoten.
13. A. Kitahara and K. Aoki, "Chemistry of Colloids and Interface" Third Ed., Hirokawa Shoten.
14. Journal of the Institute of Petroleum, Vol.54, No.539.
Return to Index